What to Expect From a Food Safety Modernization Act Inspection
The Food Safety Modernization Act (FSMA) rollout has been hanging over the specialty crop industry since the legislation was signed into law in 2011. The largest overhaul of the food safety system in more than 80 years has lived up to its billing as regulators and stakeholders have pored through the details, moving the law from development to enforcement.
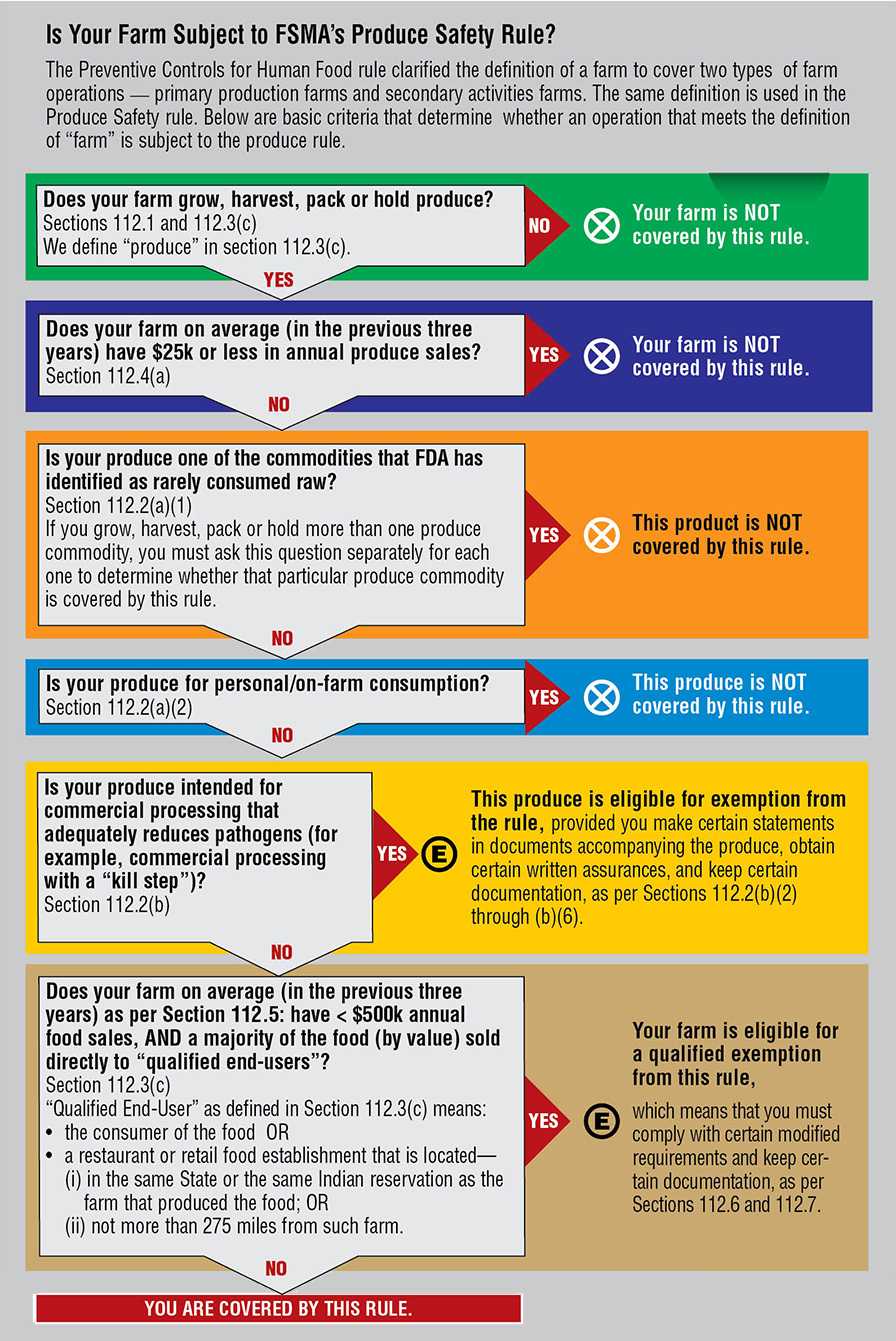
The whole idea behind FSMA is transforming food safety from a reactionary system to a preventative structure. With the vast diversity of the fruit and vegetable industry, that is no small task. That’s why the enforcement of FSMA’s Produce Safety Rule (PSR), which will govern specialty crops, has been so long in coming. But it is coming, and starting this spring.
Scheduling Inspections
According to FDA, most of the time, inspections will be scheduled in advance at a time that works for the personnel responsible for food safety on your farm and the inspector. During this pre-inspection call, the investigator will ask questions to determine if the PSR applies to your farm. Questions covered would include the size of the operation, what is grown on the farm, and how the produce is handled and/or processed.
In most cases, inspections will be scheduled within five business days of contact with the person in charge of the operation and/or the food safety point person.
While most inspections will be announced, there could be instances where they are not announced. Here are some examples of when an unannounced inspection could take place:
- If your farm has had produce safety issues in the past and the issues have not been corrected;
- If a follow-up inspection is needed, an unannounced inspection may work best to observe the necessary changes being made;
- If your farm is unresponsive (no contact within five business days after reasonable contact attempts have been made) or is unwilling to set a date for the inspection; or
- In response to a complaint, recall, or foodborne outbreak investigation.
Day of Inspection
When the inspector arrives on your farm, he or she will ask to speak to the owner or person in charge. After introductions, the investigator will show identification. It is important that the personnel in charge of food safety is available for the inspection –– or someone in authority and knowledgeable of growing, harvesting, packing, and holding processes.
The length of the inspection will vary by operation, based on size, farm activities, and what is observed during the inspection. The inspector will want to see the farm in operation by conducting a walk-through of fields, packing, storage, and other facilities. He or she will take notes, may take pictures, may collect samples, and will review and may copy records (such as training and biological soil amendment records).
According to Michelle Danyluk, an Associate Professor of food microbiology and safety with UF/IFAS, the FSMA inspection will differ from the third-party audits that many growers are accustomed to.
“Certainly, being audited for food safety means that growers are proactively addressing food safety on their farms,” she says. “Over the past few years, many of the audit programs have worked toward aligning their requirements with those of FSMA, which will help move growers toward readiness. But the reality remains that a food safety inspection isn’t an audit. The FSMA inspection will be a much more conversational approach with the walk through of the farm operation, observing potential hazards and processes.”
Exit Interview
When the walk-through is complete, the inspector will go over any regulatory concerns and findings and provide you with information on resources and technical assistance. If you are able to make corrections during the inspection, the inspector will document them. If the deficiency cannot be corrected during the inspection, the inspector will work with you to determine a reasonable time frame to implement preventive measures and corrective actions.
There are many informational resources on FSMA available on FDA’s website at FDA.gov.










